
Students then use the Vernier Emissions Spectrometer to obtain the spectral lines of helium.

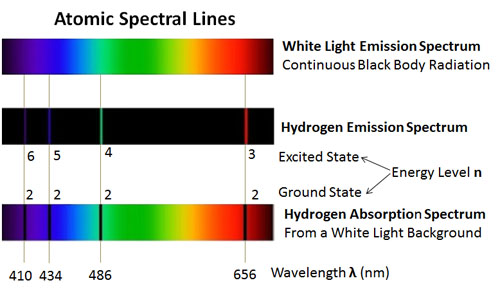
We will assume that the student has used the Vernier Emissions Spectrometer and has obtained the following lines for the visible hydrogen spectrum: 656, 486, 434, and 410 nm. Therefore, students need to collect data on the visible lines of hydrogen first or, if they have done Vernier’s Experiment 21, “Spectrum of Atomic Hydrogen,” they can use that data. The Abramzon-Siegel experiment on the helium spectrum derives its value by comparing spectral lines from the hydrogen and helium spectra. Some changes by Born in the analysis are noted, and a discussion of some of the atomic physics concepts learned is included. What follows is a description of how the Abramzon-Siegel experiment was performed with the Vernier Emissions Spectrometer. Nevertheless, this experiment would be a great addition to students studying atomic physics at the high-school level, making this experiment available to an even larger audience. Abramzon and Siegel also indicate that this experiment is ideal for college physics laboratories. This fact opens up the excellent Abramzon-Siegel helium spectrum experiment for many more students to perform. However, it has been found that a successful experiment can indeed be performed with the Vernier Emissions Spectrometer at about one-sixth the cost, even though its typical wavelength accuracy is given as ☒ nm. They indicate that for the experiment to be successful, an accuracy of ☐.5 nm is desirable. Their spectrum data was collected by the use of an Ocean Optics ™ HR4000 High Resolution Spectrometer, with a price beginning at $4,850 (as of a 6-June-2013 online catalog). Being the second simplest of atoms, helium could certainly be a starting point for a quantitative spectral analysis beyond atomic hydrogen.Ībramzon and Siegel have described a spreadsheet that allows students to perform an introductory analysis of the helium spectrum ( American Journal of Physics 77 (10), October 2009, pp. In addition, students may also be asked to identify energy level transitions by consulting tables such as the online Atomic Spectra Database compiled by the National Institute of Standards and Technology (NIST).īut, would it be possible for students to perform a more quantitative analysis of atomic spectra beyond that of hydrogen? The addition of one more proton to the nucleus and one more orbiting electron, to make helium, results in a significantly more complex atomic system. Students also generally observe that some lines are brighter than others and may classify their intensity as strong, medium or weak. These are typically studied qualitatively with students noting many more spectral lines, but with each spectrum having its unique characteristic lines.
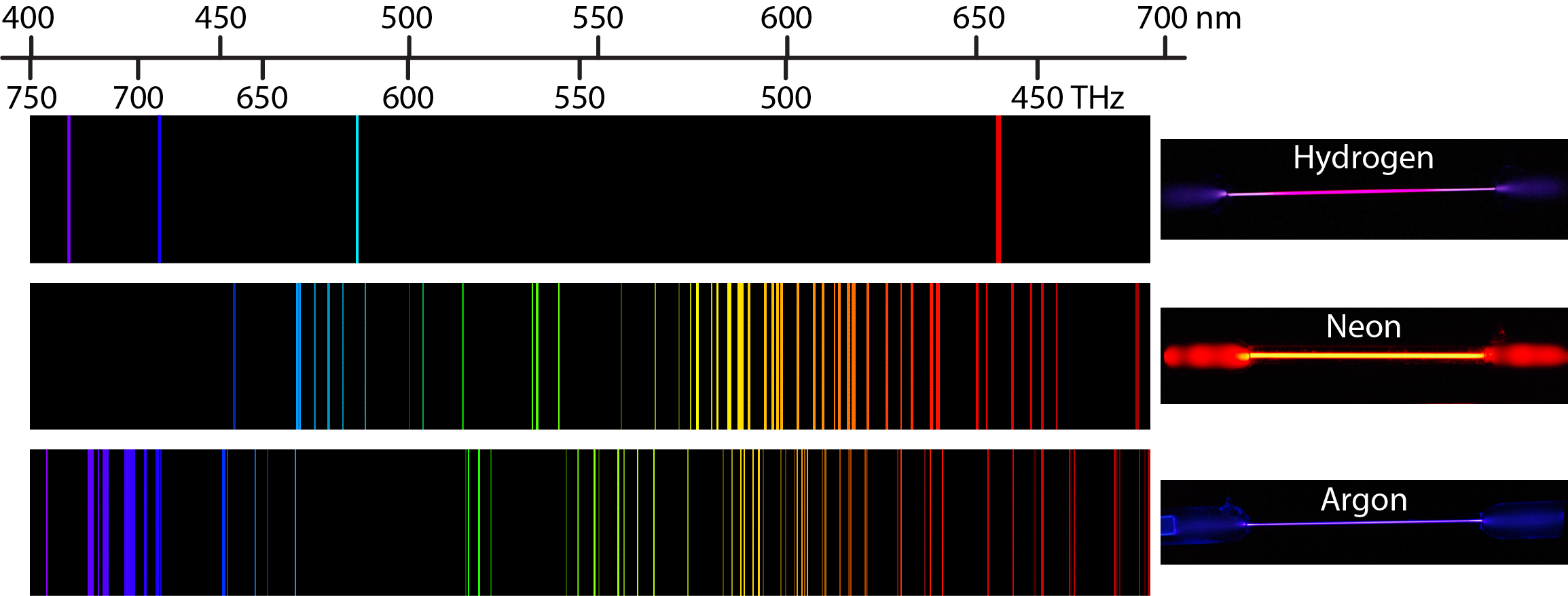
Vernier has a variety of additional spectrum tubes available including helium, nitrogen, neon, carbon dioxide, air and argon. In this experiment, students use the Vernier Emissions Spectrometer to determine the wavelengths of the visible lines of excited hydrogen gas, relate photon energies to energy level transitions, and determine a value for the Rydberg constant for hydrogen.
#Atomic spectra of neon series#
The “Spectrum of Atomic Hydrogen,” Experiment 21 in Advanced Physics with Vernier–Beyond Mechanics, is a classical investigation of the Balmer Series of the hydrogen spectrum.


 0 kommentar(er)
0 kommentar(er)
